Vapor Liquid Equilibrium
Vapor-liquid equilibrium (known as VLE) is the state in which the rate of condensation is equal to the rate of evaporation so there is no net vapor-liquid interconversion (Wikipedia). Typically, a substance in vapor-liquid equilibrium is at its boiling point.
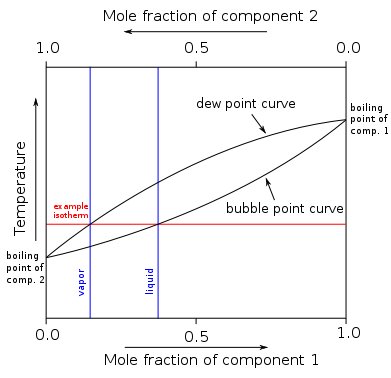
A boiling point diagram for a binary mixture is found to the right. For a range of temperatures, the mole fraction of a component in the vapor phase is found along the dew point curve while the mole fraction of the component in the liquid phase is found along the bubble point curve.
Vapor-liquid equilibrium of a mixture can be modeled by Raoult's Law. Developed in 1882 by Francois-Marie Raoult, it states that the partial vapor pressure of each component of an ideal mixture is equal to the mole fraction of the component in the mixture multiplied by the vapor pressure of the pure component (Wikipedia). The total vapor pressure of the solution can then be determined by the sum of the partial pressures of each component.
Raoult's Law
Want more videos like this? Click here to see more on vapor liquid equilibrium.
For more on Raoult's Law, UC Davis' ChemWiki has some great information here.

 Online Courses
Online Courses